article image source: phys.org (Link)
Amino Acid “Stickers” Unlock the Secret to Spider Silk’s Strength and Flexibility
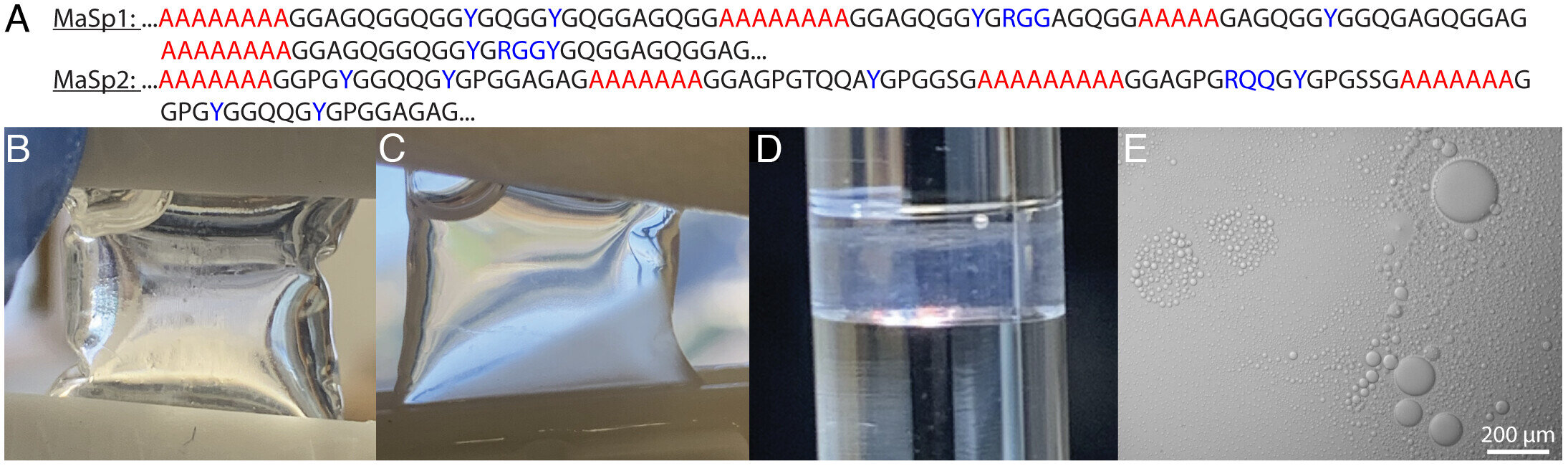
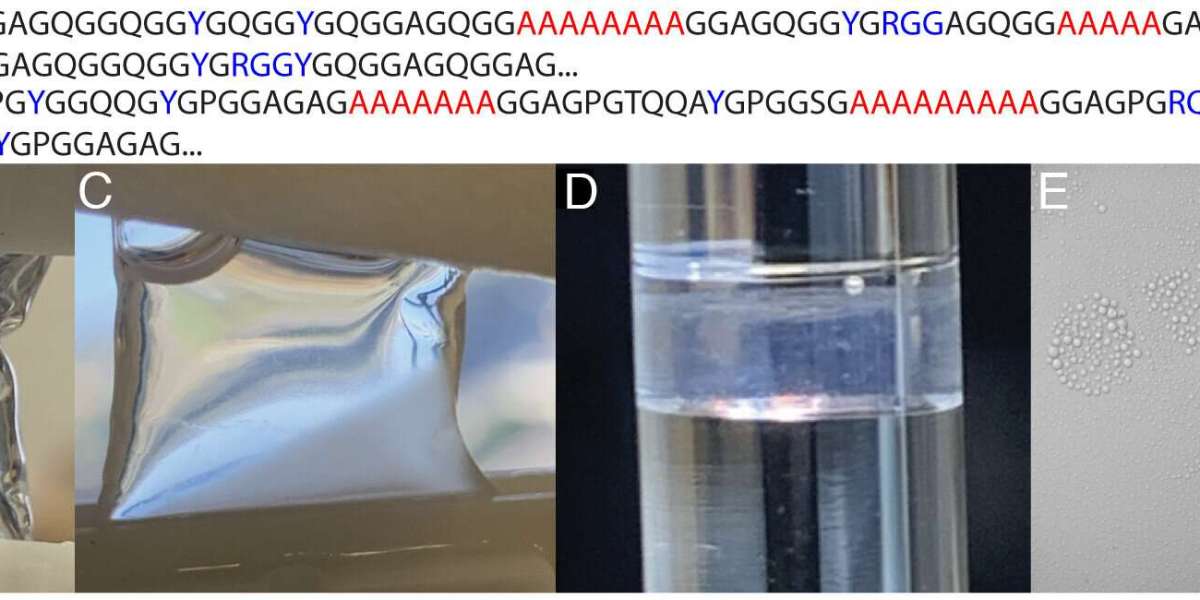
Phosphate induces LLPS in native MaSp. (A) Consensus sequence for L. hesperus MaSp1 and MaSp2. Credit: Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2523198122
image source: phys.org
Key Points:
Spider silk proteins use molecular “stickers” for incredible strength and stretch.
Arginine and tyrosine amino acids drive fiber formation and nanostructure.
Insights from silk could inspire sustainable materials and advance neurological research.
advertisement
Spider silk has long fascinated scientists for its remarkable combination of strength, flexibility, and toughness—stronger than steel by weight and tougher than Kevlar. Now, groundbreaking research from King's College London and San Diego State University has unveiled the molecular interactions behind this natural marvel. Their findings reveal that certain amino acids act as molecular “stickers,” orchestrating the assembly of silk proteins into high-performance fibers.
The study, published in the Proceedings of the National Academy of Sciences, highlights that arginine and tyrosine amino acids play a pivotal role. They first drive the initial clustering of proteins in the spider’s silk gland, where silk proteins are stored as a concentrated liquid called “silk dope.” These same interactions persist as fibers are formed, creating the complex nanostructure that gives spider silk its extraordinary mechanical properties.
Using advanced techniques—such as molecular dynamics simulations, AlphaFold3 modeling, and nuclear magnetic resonance spectroscopy—the interdisciplinary team provided an atomistic explanation for how disordered proteins can self-organize into highly ordered, resilient structures.
Professor Chris Lorenz of King's College London emphasizes the potential of these insights: “Lightweight protective clothing, airplane components, biodegradable medical implants, and even soft robotics could benefit from fibers engineered using these natural principles.”
Interestingly, the study also suggests connections to human health. According to Professor Gregory Holland of SDSU, “The way silk proteins undergo phase separation and form β-sheet–rich structures mirrors mechanisms in neurodegenerative diseases such as Alzheimer’s. Studying silk gives us a clean, evolutionarily optimized system to understand how these processes can be controlled.”
In essence, spider silk is not just a natural wonder—it is a model for designing the next generation of sustainable, high-performance materials, while also offering a window into fundamental biological processes that impact human health.
Conclusion:
The discovery of molecular “stickers” in spider silk opens exciting possibilities beyond biomaterials. From aerospace and protective gear to medical implants and neurological research, these findings exemplify how understanding nature at the molecular level can transform technology and medicine. Spider silk continues to inspire innovation, proving that even the smallest amino acids can teach us big lessons about strength, flexibility, and the elegance of natural design.
Key Points Summary:
Arginine and tyrosine amino acids act as molecular “stickers” in spider silk.
These interactions drive protein clustering and fiber formation.
Insights could lead to high-performance, sustainable fibers and advance neurological research.
advertisement
Frequently Asked Questions (FAQ):
Q1: What makes spider silk stronger than steel?
Spider silk’s strength comes from its nanostructure, formed by amino acids like arginine and tyrosine that act as molecular “stickers,” organizing proteins into tightly packed, resilient fibers.
Q2: How are spider silk proteins formed?
Silk proteins are stored in the spider’s silk gland as a concentrated liquid called “silk dope.” They cluster via amino acid interactions and then solidify into fibers during spinning.
Q3: Can we make synthetic spider silk?
Yes! Understanding the molecular interactions in natural spider silk provides design principles for creating bio-inspired, high-performance fibers for clothing, aerospace, and medical applications.
Q4: Does spider silk research have medical implications?
Absolutely. The phase separation and β-sheet formation in silk proteins mirrors processes in neurodegenerative diseases like Alzheimer’s, offering a model for study and potential therapies.
Q5: What are the potential applications of this research?
Applications include lightweight protective gear, airplane components, biodegradable medical implants, soft robotics, and materials inspired by nature’s efficiency and strength.
Sources:
Phys.org – Overview of spider silk molecular interactions and applications
https://phys.org/news/2026-02-amino-acid-stickers-decode-spider.html
Thank you !