article image source: dementiasplatform.uk (link)
In a historic medical breakthrough, researchers have successfully treated Huntington’s disease for the first time, dramatically slowing its progression by 75%. This major step forward offers new hope for individuals and families affected by one of the most devastating genetic disorders.
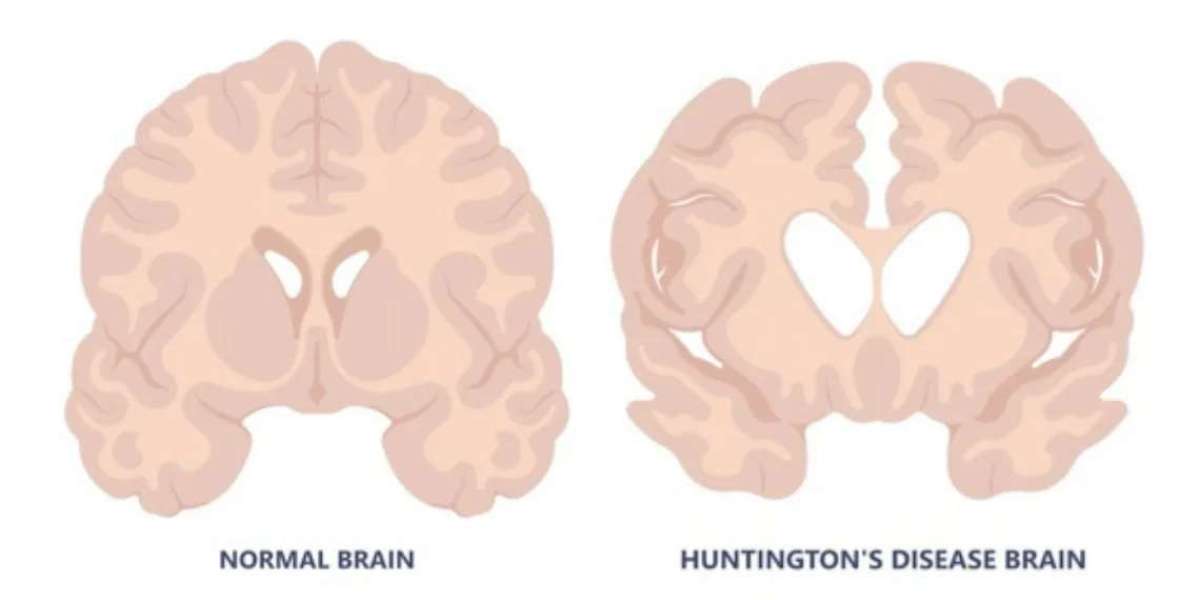
Huntington’s disease, an inherited condition that typically appears in a person’s 30s or 40s, damages the brain and combines symptoms seen in dementia, Parkinson’s, and motor neurone disease. It's often fatal within 20 years of diagnosis.
Researchers at University College London (UCL), led by Prof. Sarah Tabrizi, conducted a pioneering clinical trial using an advanced form of gene therapy. The treatment involved a single, highly complex brain surgery lasting up to 18 hours. During the procedure, a modified virus delivered genetic instructions into two key brain regions. These instructions helped brain cells produce microRNA designed to suppress the harmful mutant huntingtin protein responsible for the disease.
Life-Changing Results for Patients
The trial, conducted with 29 patients, showed a remarkable 75% slowdown in the disease’s clinical progression over three years. For patients, this means a decline that would usually occur in one year would now take four. In real terms, individuals expected to be in wheelchairs are still walking, and one patient has even returned to work after early retirement.
Prof. Ed Wild of UCLH described the results as “breathtaking,” noting that they go far beyond what anyone had hoped for. The treatment also preserved brain cells, as shown by reduced levels of neurofilaments – markers of cell death – in spinal fluid.
While some side effects such as inflammation and headaches were reported, these were temporary or managed with steroids. The treatment is believed to be long-lasting, as brain cells aren’t replaced in the same way as other tissues.
advertisement
Looking to the Future
Though the therapy offers incredible promise, its rollout will be limited initially due to the complexity and cost of the surgery. uniQure, the biotech firm behind the treatment, plans to seek approval in the U.S. in early 2026, with other regions to follow.
Prof. Tabrizi called the trial “the beginning” of a new era in Huntington’s treatment. She’s already working on a prevention study targeting young individuals who carry the gene but have not yet developed symptoms — with the hope of delaying or even stopping the disease before it begins.
For people like Jack May-Davis, who carries the Huntington’s gene and has watched family members suffer, the news is life-changing. “It does allow me to think my life could be that much longer,” he said.
Thank you !